11th Summit on Hematologic Malignancies, April 2-7, 2024
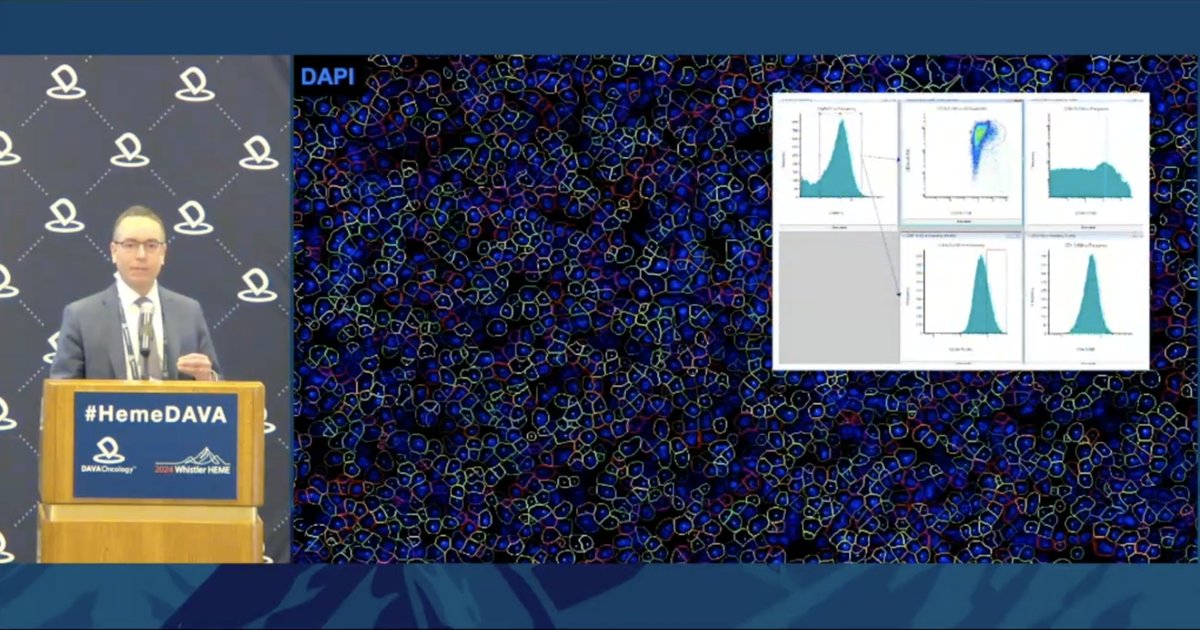
Dr. JC Villasboas @MayoClinic shares multiplex immunofluorescence system combining histology, immunohistochemistry, and high-parameter flow cytometry, all obtained from a single section of tissue while preserving the spatial organization of the tumor.
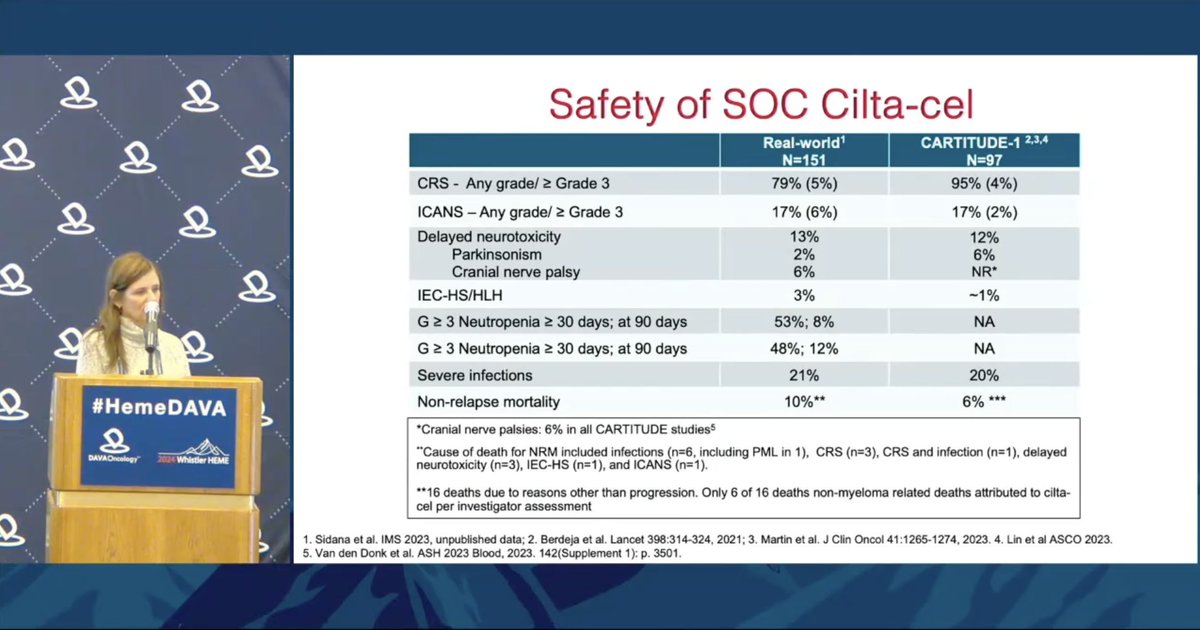
Dr. Melissa Alsina @MoffittNews shares RWE for cilta-cel ➖57% of pts are not trial eligible, but response rates similar to CARTITUDE-1 ➖Safety similar to trial, w/ delayed neurotox in 13% ➖Significant association between CRS and BM plasma cells ≥50% and ECOG 2-4.
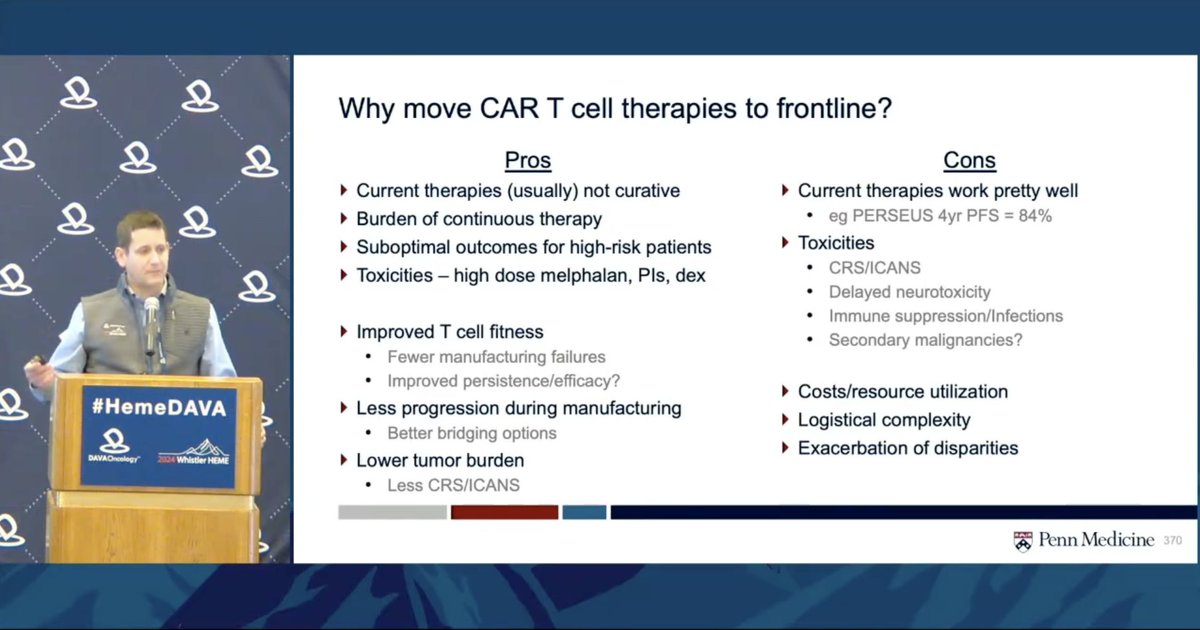
@CohenAd_MMdoc reviews the data and rationale for moving CAR-T to frontline MM, with a nice overview of the pros and cons PERSEUS already provides 84% PFS at 4 years What additional efficacy benefit would you need to see from CAR-T to balance the cost and potential toxicity?
We’ve got BCMA CAR-T, BCMA BsAbs, GPRC5D BsAbs... and now GPRC5D-targeting CAR-Ts are in development. An abundance of riches, but now we must address the question of sequencing @DoctorAKrishnan asks our panel where will these agents fit into our MM treatment paradigm?

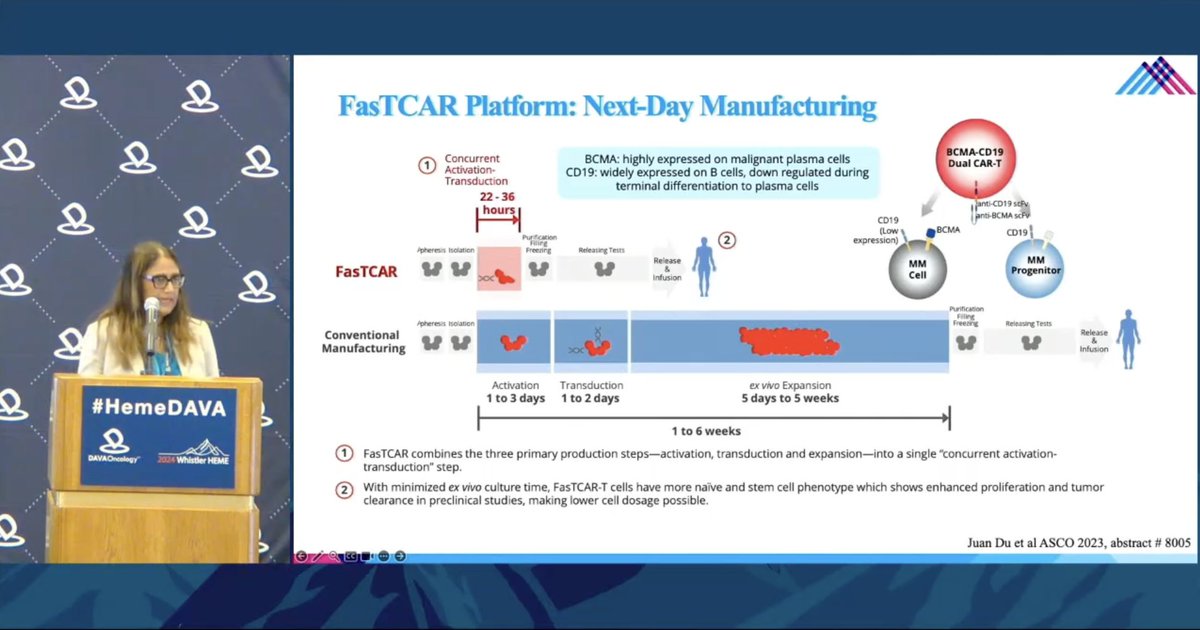
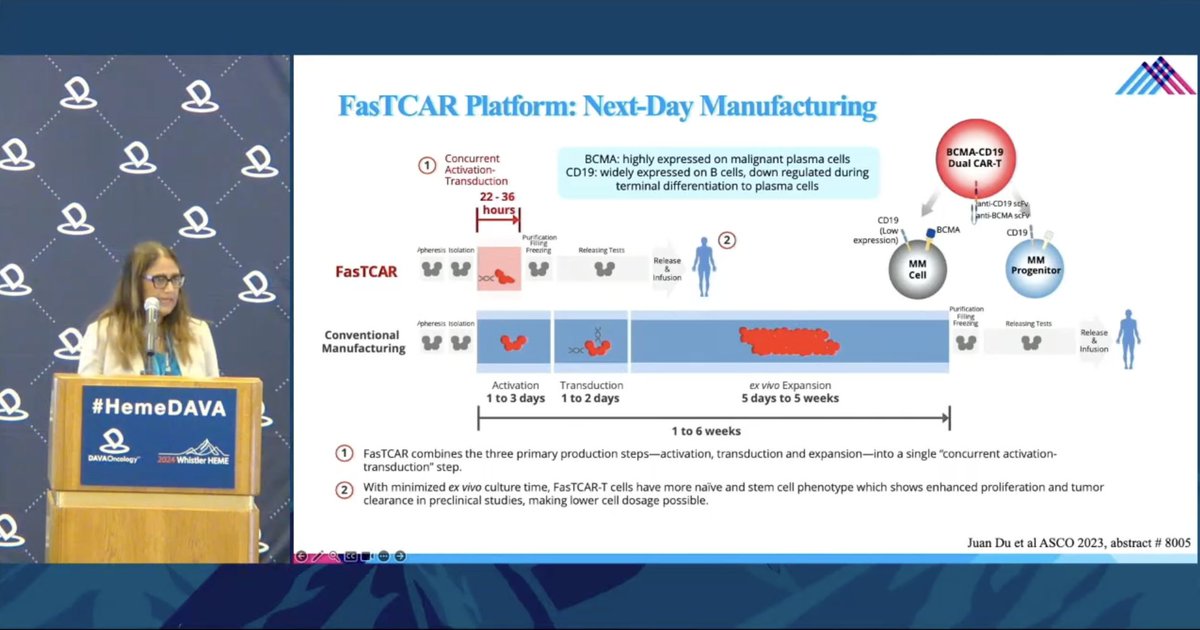
Dr. Shambavi Richard @IcahnMountSinai shares exciting new technology with a dual targeting BCMA-CD19 CAR manufactured w/ next-day FasTCAR platform Improving the speed and success rate of CAR-T manufacturing is key maximize patient access to these therapies.
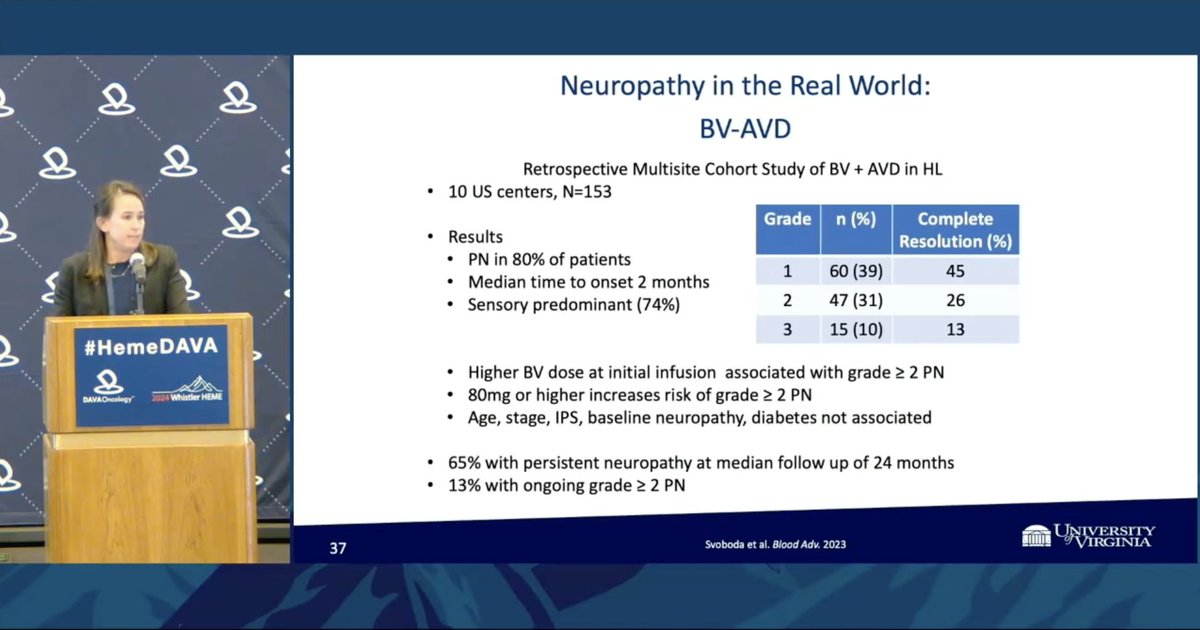
Dr. Emily Ayers presents RWE for peripheral neuropathy with BV-AVD in HL patients ➖80% of patients developed PN ➖Higher BV dose (≥80mg) at initial infusion associated with grade ≥2 PN ➖Interestingly, diabetes and baseline PN not associated w/ incidence or severity of PN.
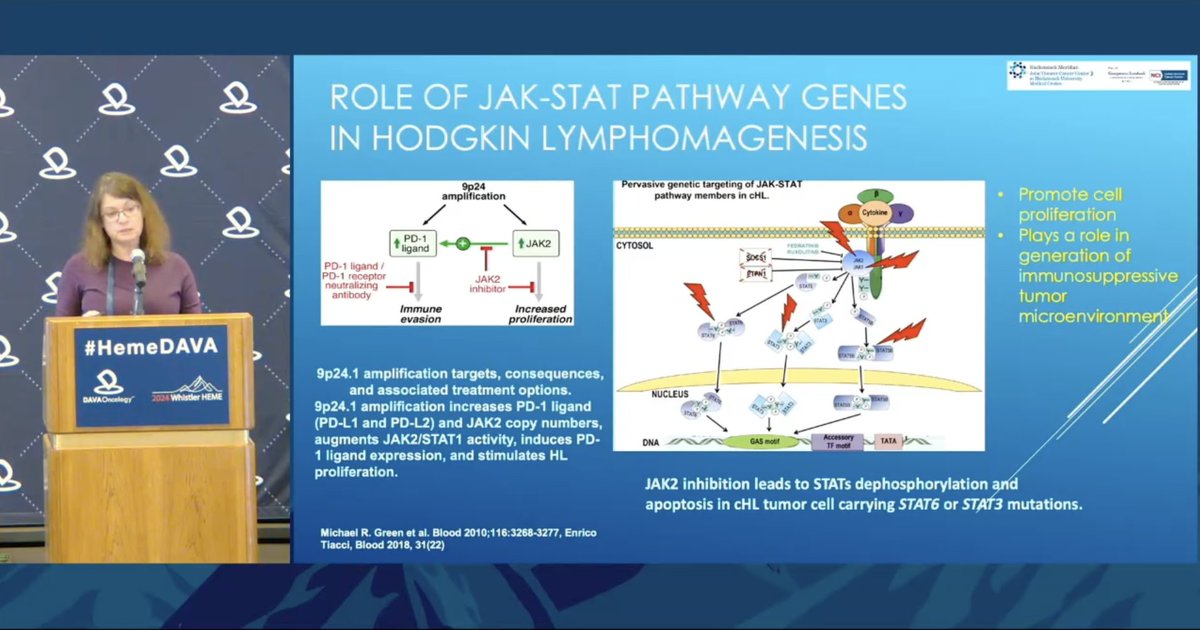
Dr. Tatyana Feldman @JTCancerCenter discusses biology of targeting JAK/STAT in HL and initial data for KT-333, a STAT3 degrader Previously considered “undruggable” target, blocking STAT3 normalizes the microenvironment in HL Phase 1a/1b trial recruiting: https://clinicaltrials.gov/study/NCT05225584.
Dr. Shambavi Richard @IcahnMountSinai shares exciting new technology with a dual targeting BCMA-CD19 CAR manufactured w/ next-day FasTCAR platform Improving the speed and success rate of CAR-T manufacturing is key maximize patient access to these therapies.
Wrapping up #DAVAWhistlerHeme with teaser from Dr. Paolo Strati @MDAndersonNews, phase 1 trial of evorpacept and R2 for treatment of pts with r/r B-NHL Evorpacept increases antibody-dependent phagocytosis in macrophages Tune in to @AACR on April 9th to see the results!
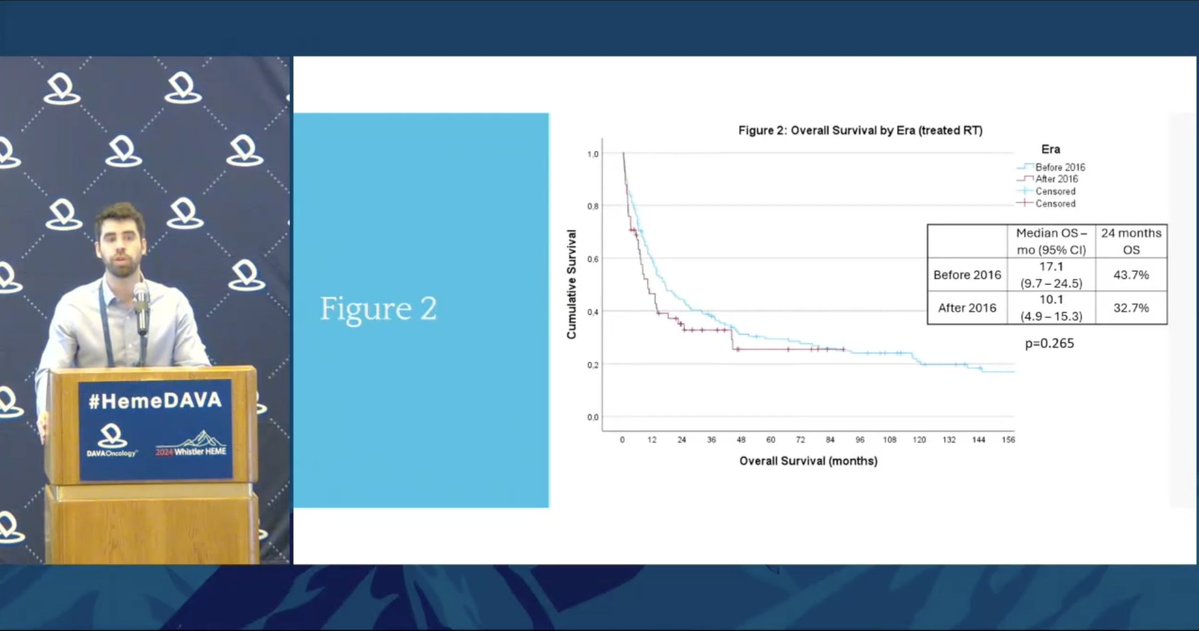
Will novel agents in CLL decrease risk of RT? Dr. Jean-Nicolas Champagne shares data from 219 RT patients, 144 pre- and 75 post-2016. Post 2016: ⬆️Time from txt to RT ⬇️PS at time of RT ⬇️OS after RT RT remains challenging. More tolerable therapies are needed in the modern era.
Dr. John Baird returns to the stage to discuss BAFF-R as a target to overcome antigen escape mechanisms of resistance DL2 currently enrolling in MCL, LBCL, FL, MZL: https://classic.clinicaltrials.gov/ct2/show/NCT05370430 Ph 1 for B-ALL also enrolling: https://clinicaltrials.gov/study/NCT04690595.
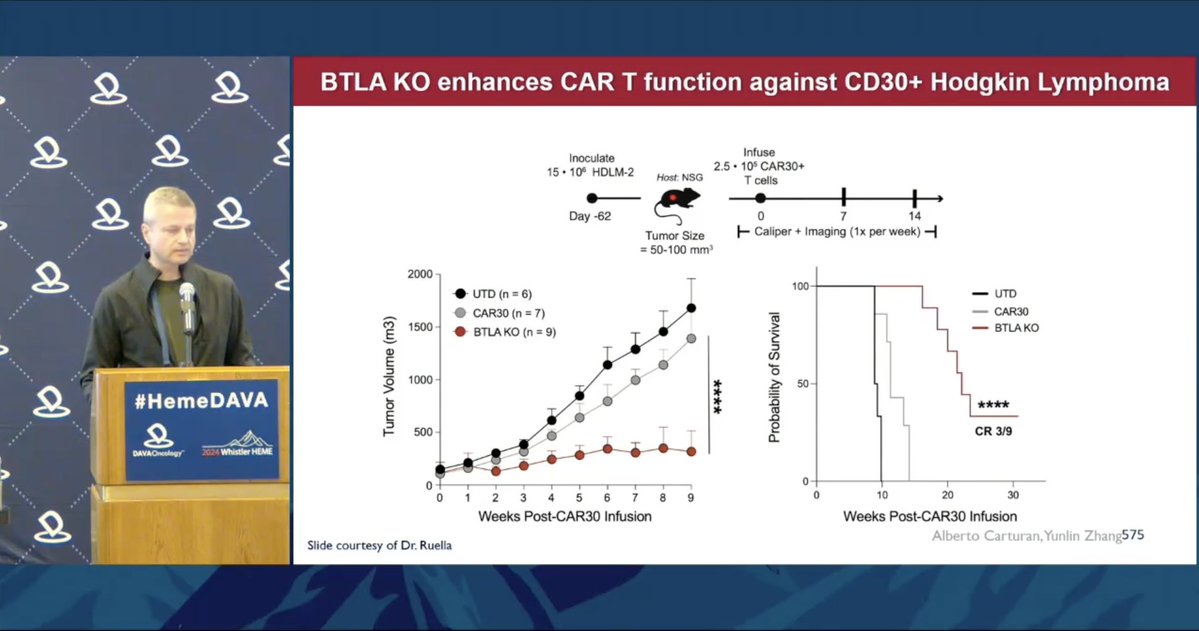
CD30 CAR-T has been disappointing in HL. Dr. Jakub Svoboda shares “engine fine tuning” w/ CRISPR knock out BTLA-HVEM, a CPI that is enriched in HL and suppresses immune activation Murine models show enhanced CD30 CAR-T function with BTLA KO, clinical trials in planning stage.
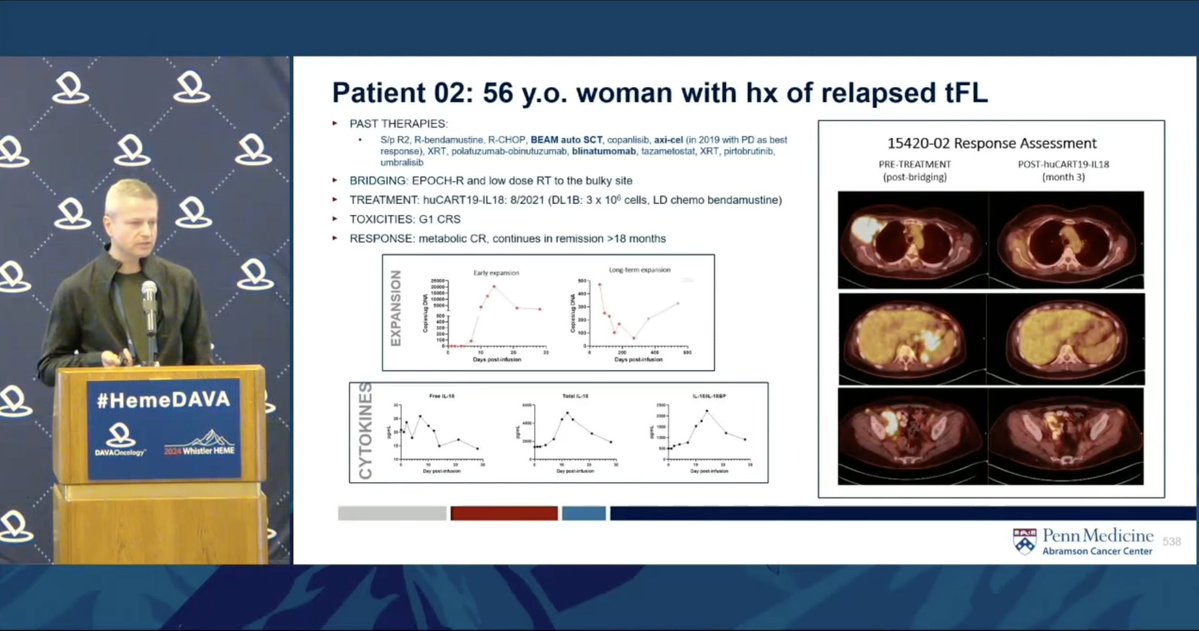
Dr. Jakub Svoboda @PennMedicine discusses armored huCART19-IL18 with encouraging early results in CD19+ lymphoma No new safety signals w/ IL-18 CRS/ICANS rates comparable, reversible 82% ORR at 3 mo.
@KrishPatel, MD reviews rationale for dual targeting CD19/CD20. CD20 is expressed independent of CD19, targeting both may improve durability of response by limiting antigen escape. C-CAR039 demonstrated deep and durable response as well as improved safety.

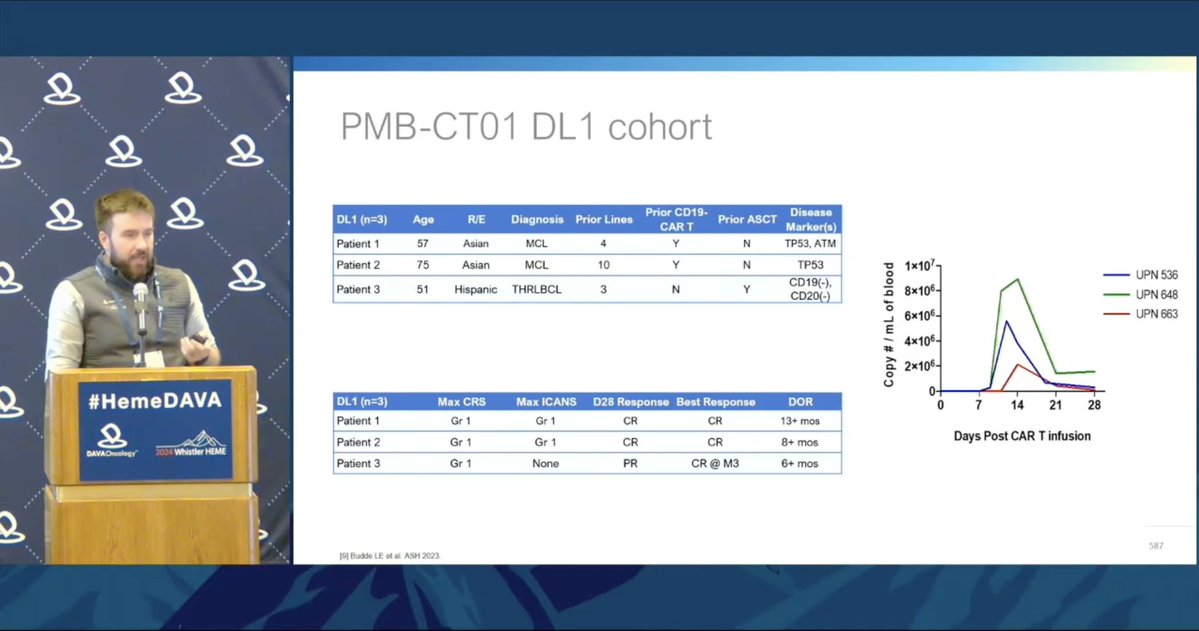
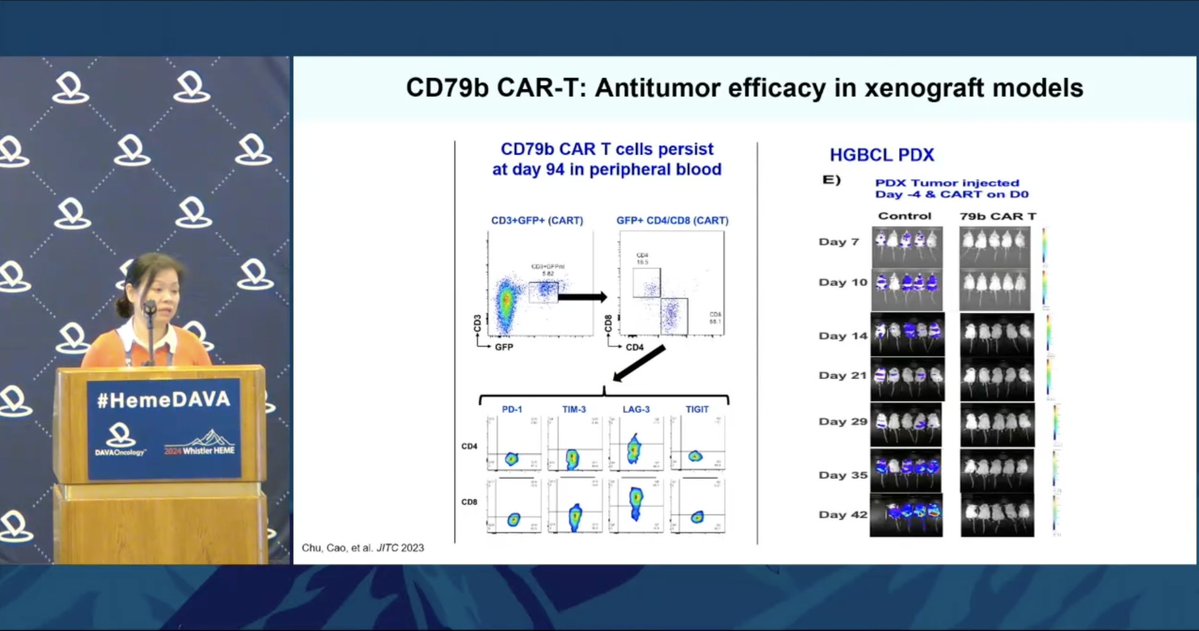
@elizabeth_budde shares preclinical data for CD79b-targeting CAR-T products CD79b is a B-cell lineage marker expressed across most B-cell NHL, independent of CD19 expression Two phase 1 trials are actively recruiting: https://clinicaltrials.gov/study/NCT05773040 https://classic.clinicaltrials.gov/ct2/show/NCT06026319.
Dr. Daniel Landsburg @Penn shares distinction between detecting a TP53 mutation and determining its functional status. DLBCL patients with TP53 LOF mutation had worse outcomes than non-LOF mutations, highlighting need for more precise molecular stratification.

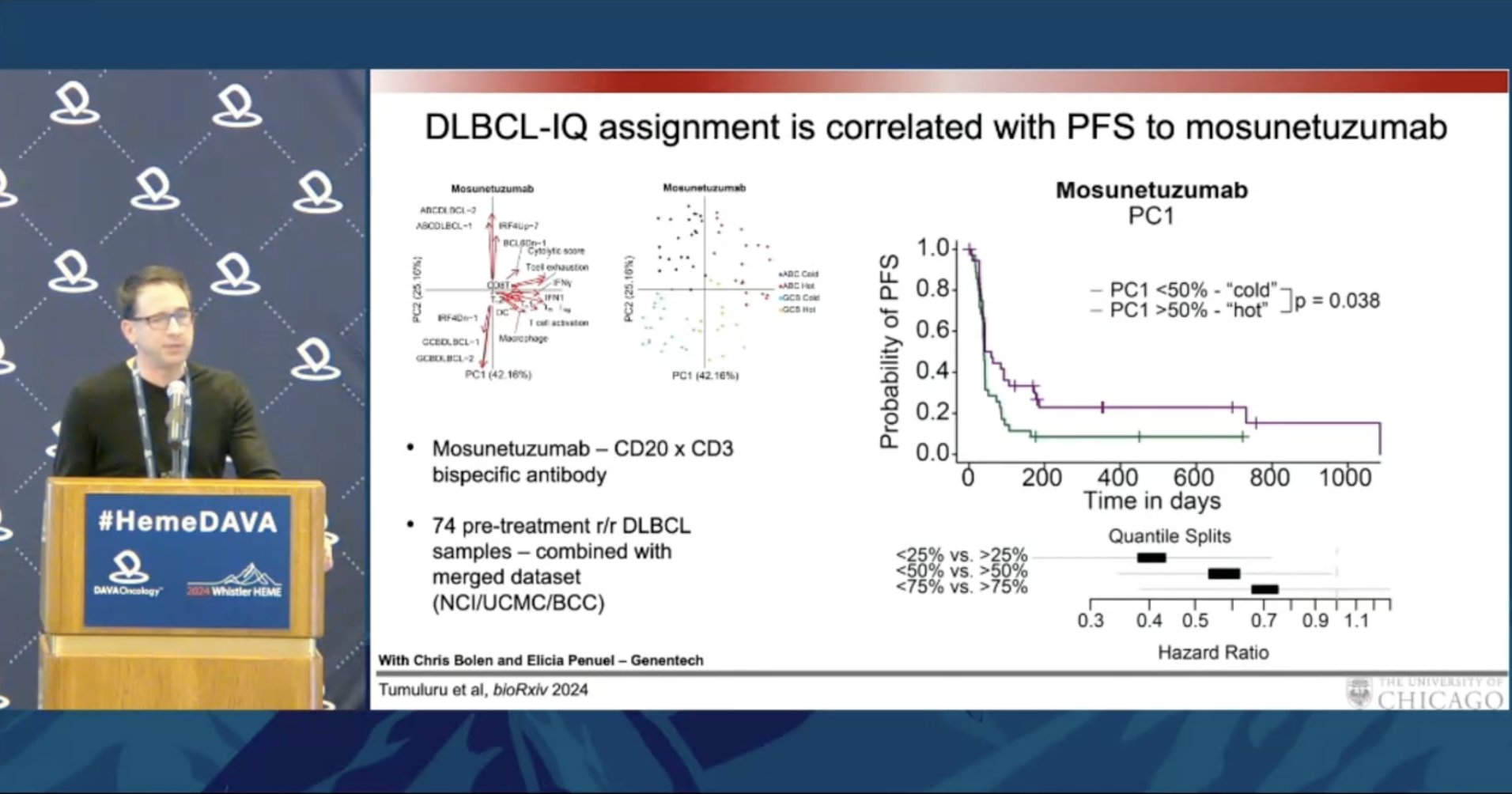
Dr. Justin Kline @UCCancerCenter shares a new DLBCL classification using immune environment characteristics. "Hot" and "cold" DLBCL may have different responses to IO. DLBCL-IQs are associated with improved PFS with mosunetuzumab, but not axi-cel.
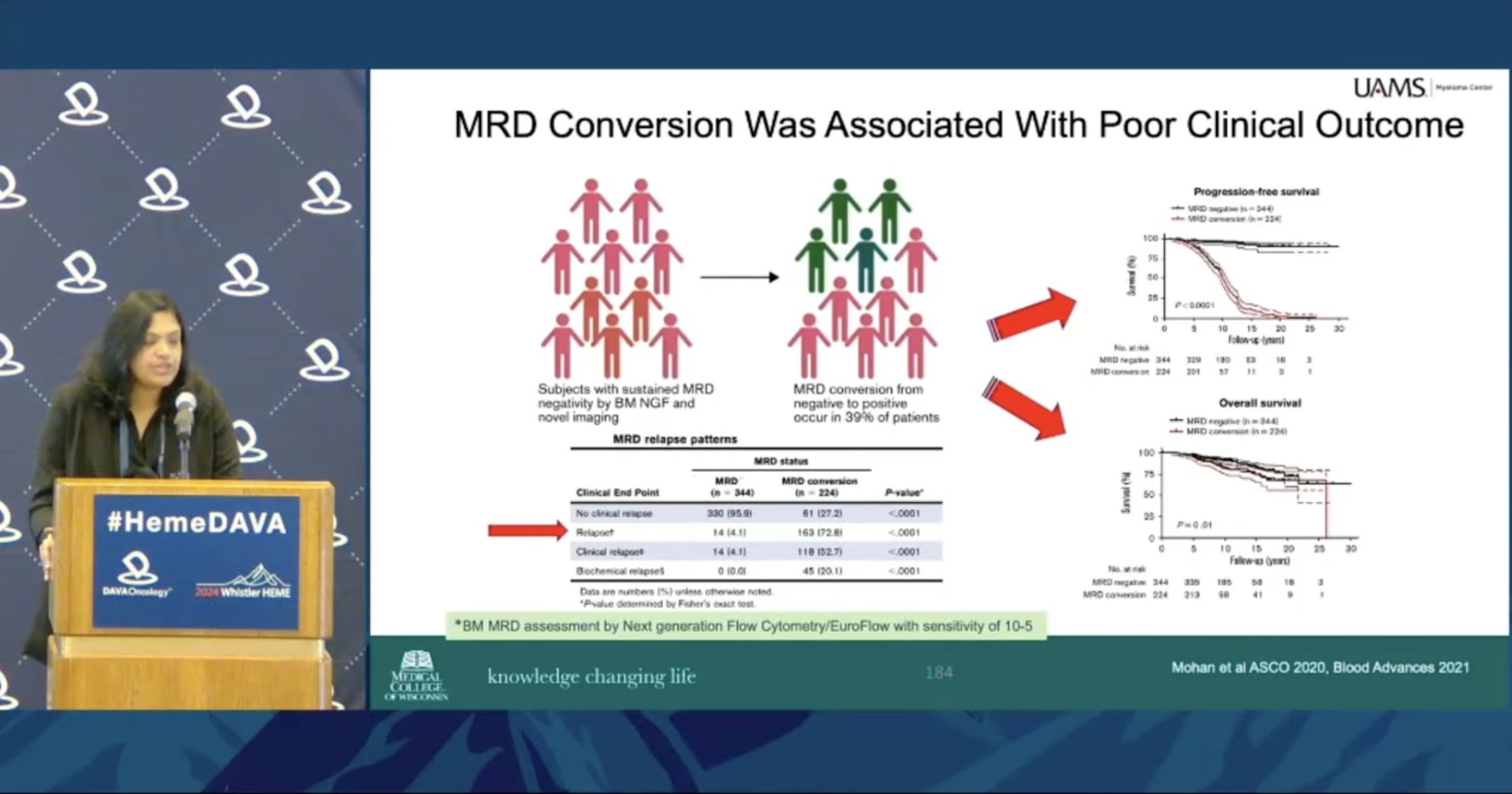
Meera Mohan MD sets the stage for upcoming MRD lectures with outcomes for patients who fail to achieve uMRD or convert from MRD negative to positive 39% of patients experience MRD conversion, which is highly predictive of relapse How do we use MRD to optimize treatment in MM?
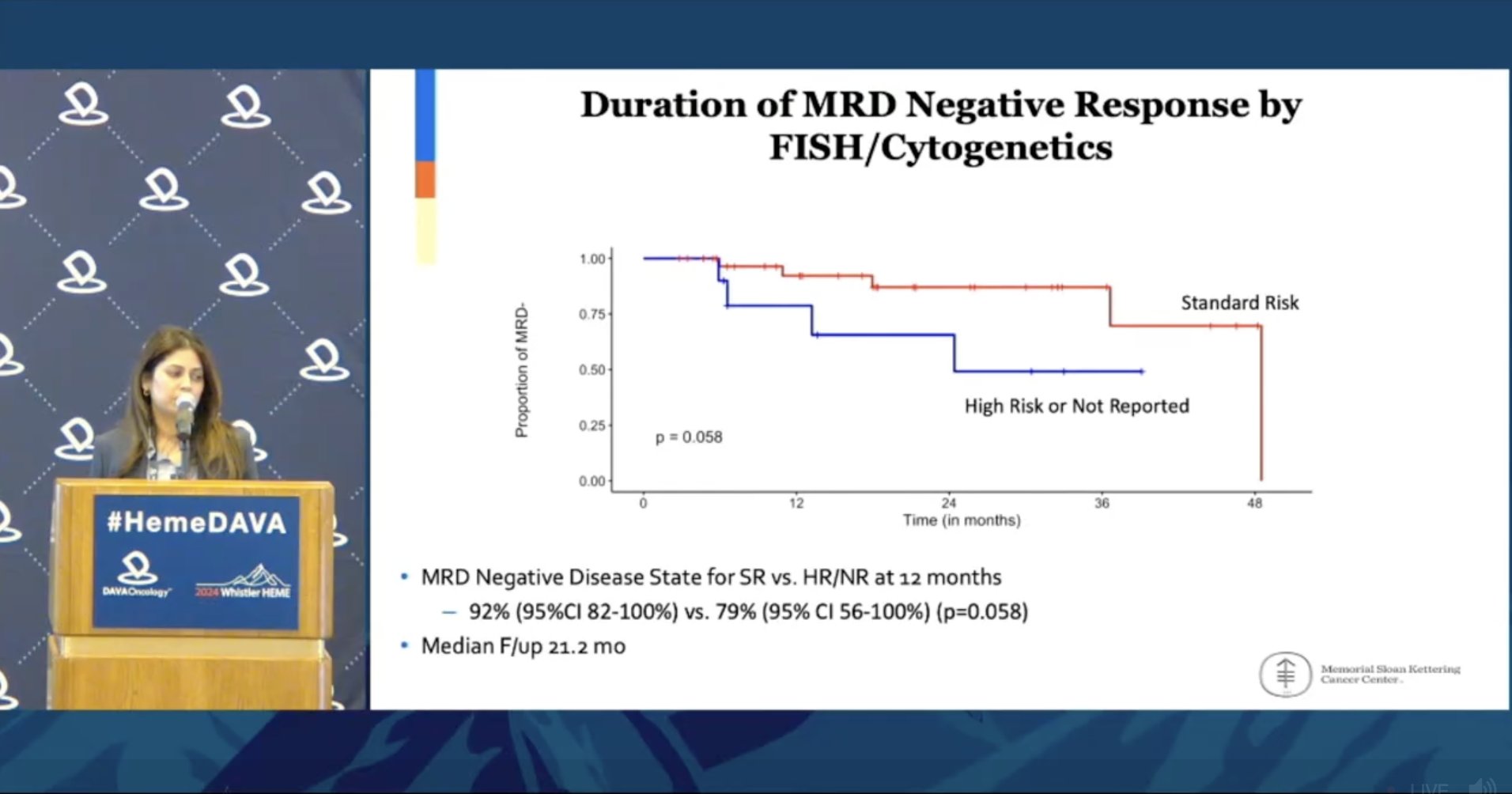
Continuing our MRD discussion @kordeneha1 shares IIT investigating outcomes if maintenance is discontinued after 3 years uMRD ➖21.2 mo median f/up ➖84% of patients remain uMRD at 12-mo ➖Cytogenetic risk impacts duration of uMRD Looking forward to phase 3 data from SWOG1803!
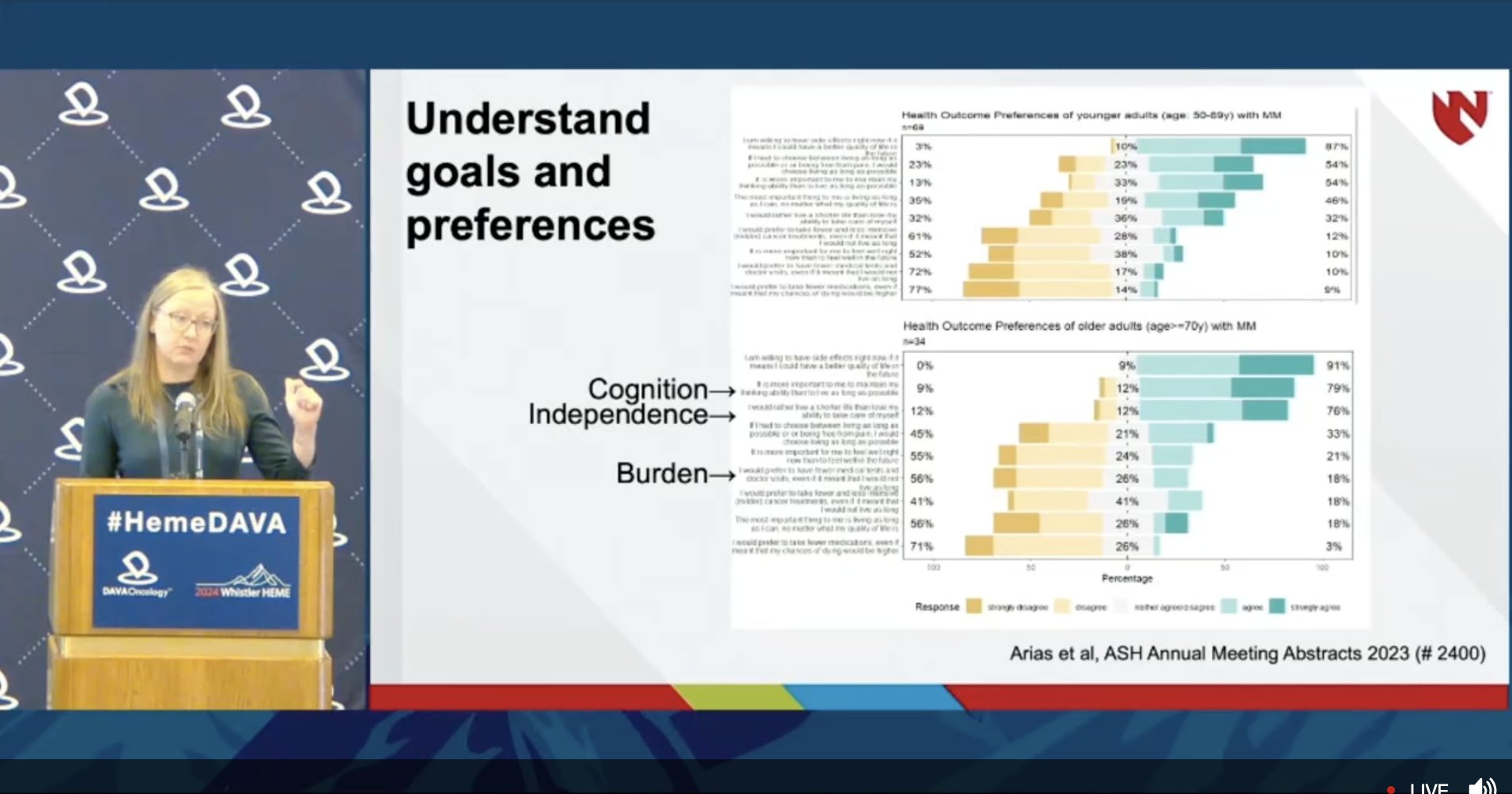
Rounding off this panel, @tanyawildes shares a practical geriatric assessment released by ASCO last year to help risk stratify geriatric patients with MM. https://society.asco.org/sites/new-www.asco.org/files/content-files/practice-patients/documents/2023-PGA-Final.pdf Cognition, independence, and burden of therapy are priorities for patients ≥70y.
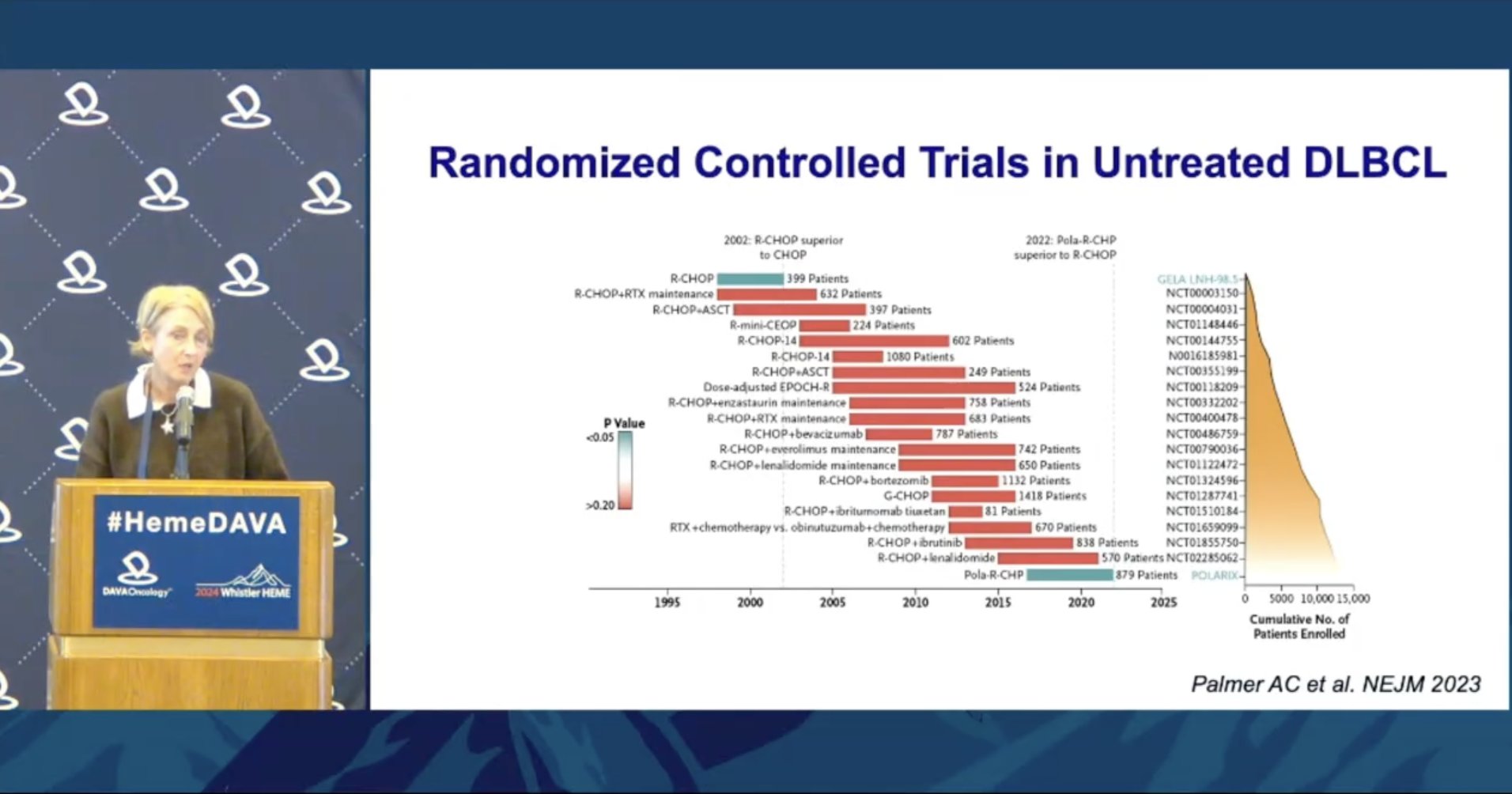
Presented by @SehnLaurie. Exciting to finally see a positive trial in frontline DLBCL! Also amazing to see the 15,000 patients in the figure to the right who participated in these trials over the years. Thank you for your invaluable contribution to medicine!
Awesome team tackling MM @DAVAOnc covering from NDMM to MM in frail.

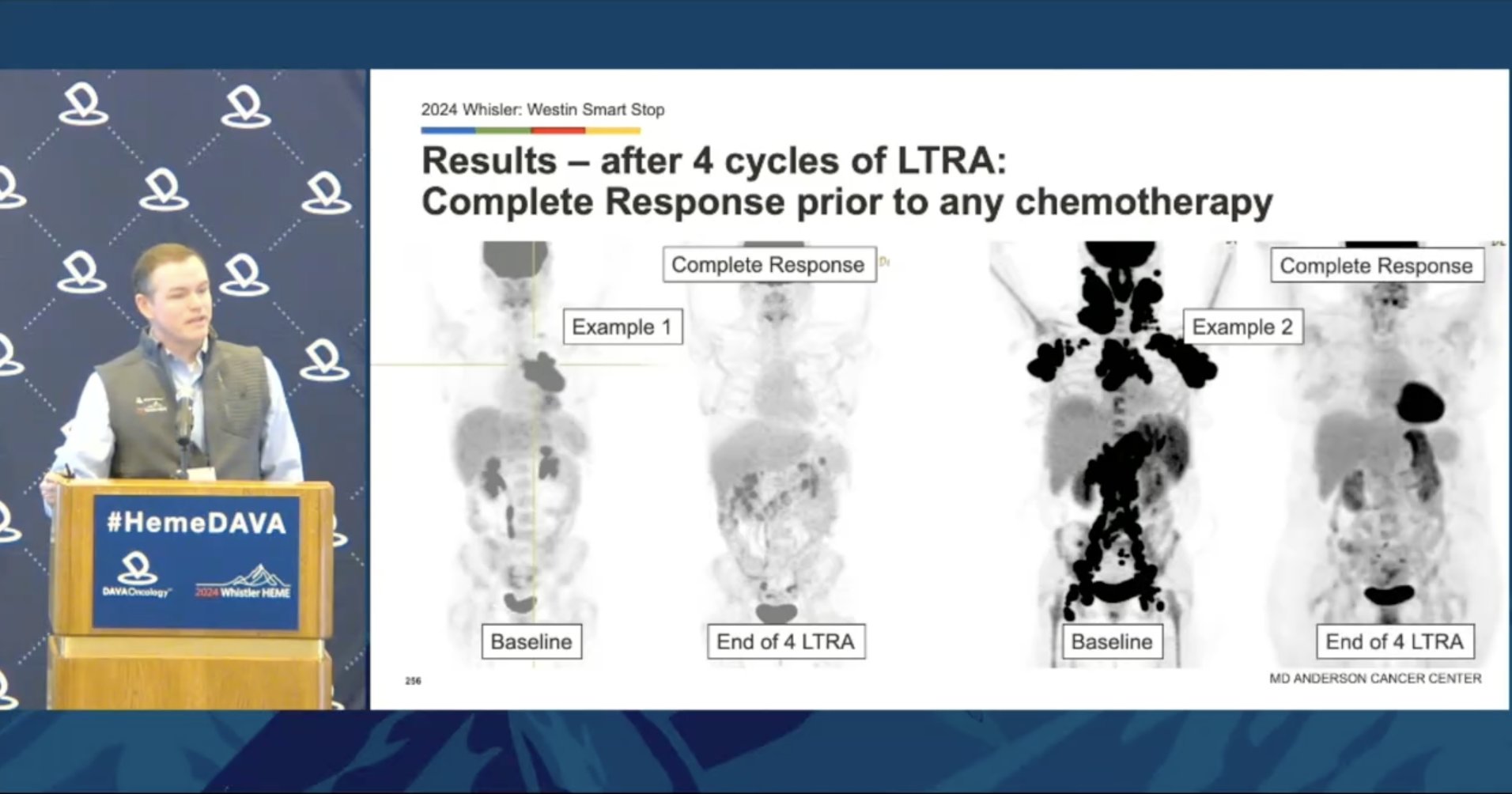
Exciting results from a potentially chemo-free treatment regimen for DLBCL presented by @Lymphoma_Doc Smart Stop uses 4 cycles of LRTA (len, tafa, ritux, acala) followed by response-guided CHOP After 4 cycles LRTA: ➖ORR 100% ➖CR 63.3% After 2 cycles of CHOP, CR is 93.3%!
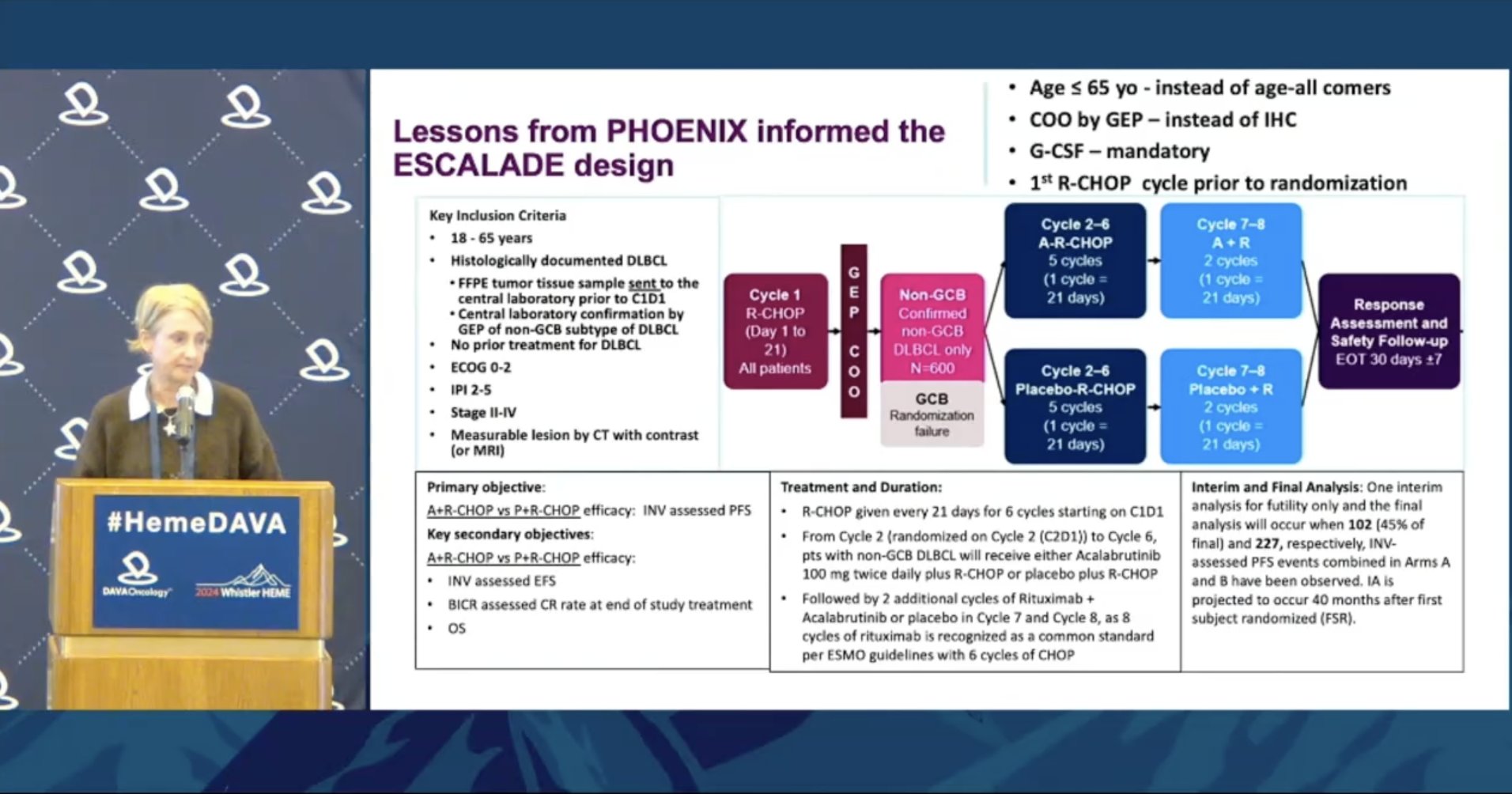
Ibrutinib had a strong signal of activity in DLBCL with an OS advantage in younger patients. Can we see even better risk/benefit profile with acalabrutinib? Looking forward to data from ESCALADE at an upcoming conference.
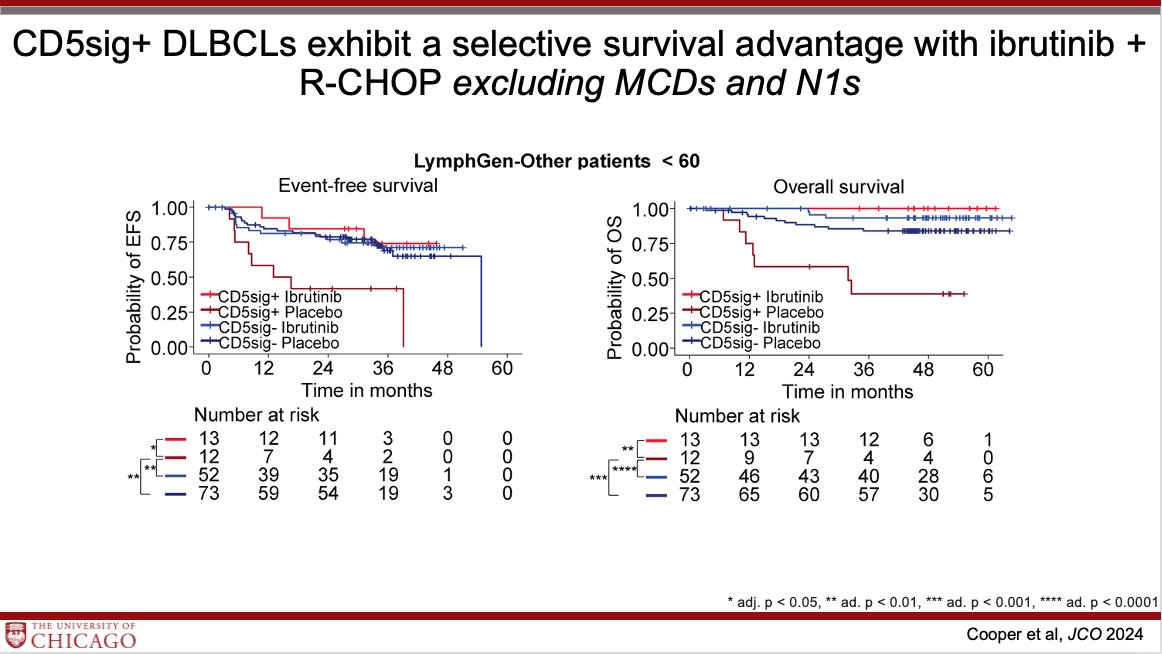
Dr. Justin Kline @UCCancerCenter discusses CD5 expression as a biomarker for BTKi response in DLBCL ➖CD5 is a marker of B cell activation ➖Enriched in non-GCB, MYD88, CD79B mutations ➖CD5+ DLBCL are BCR-driven and respond to ibrutinib + R-CHOP.
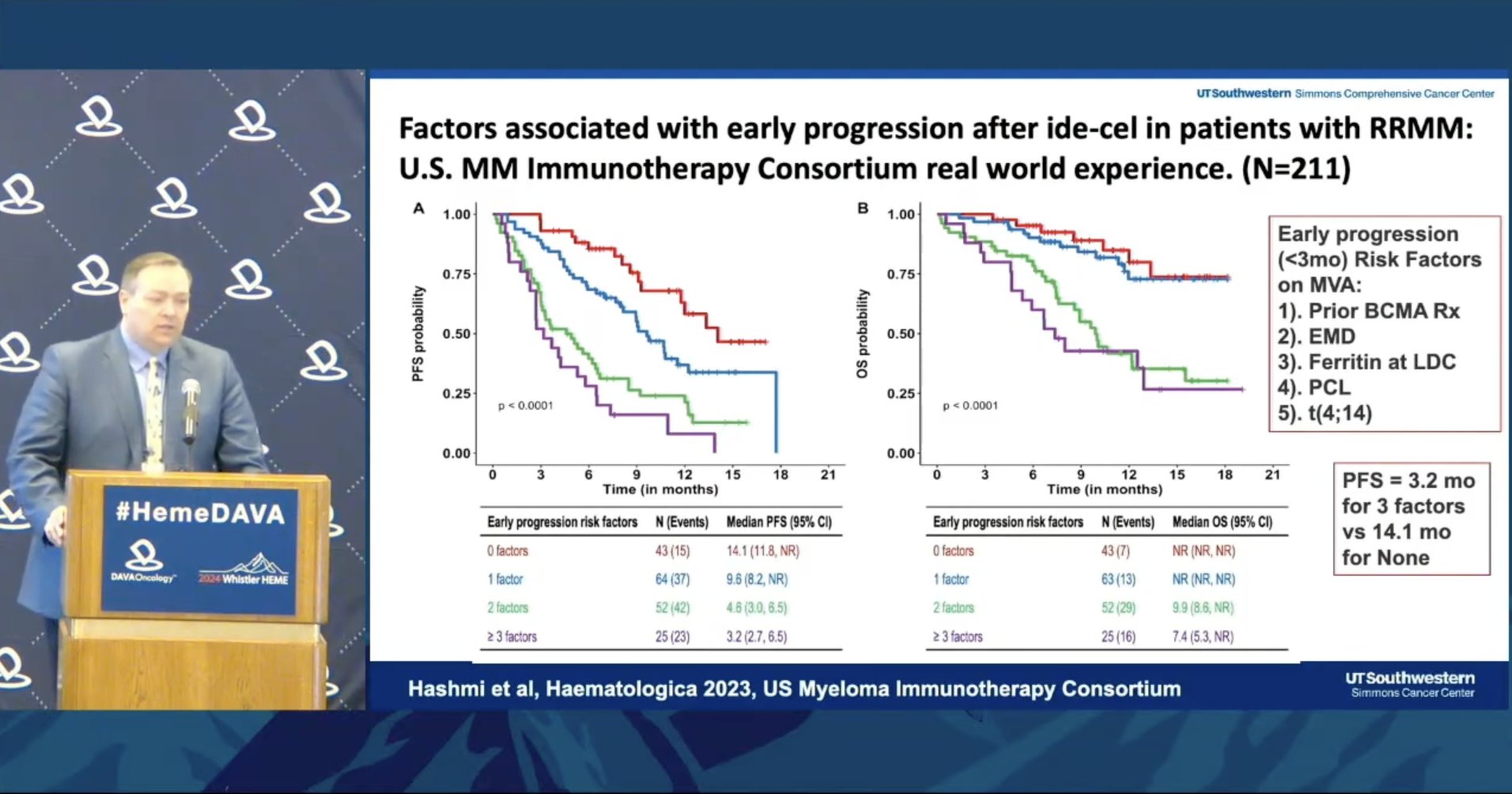
Kicks off cellular therapies in MM session with RWE for ide-cel Early progression (<3 mo) after ide-cel associated with: ➖Prior BCMA therapy ➖EMD ➖Ferritin at LDC ➖PCL ➖t(4;14) Specifically, prior BCMA BsAb and ADC (not CAR-T) had negative impact on response.
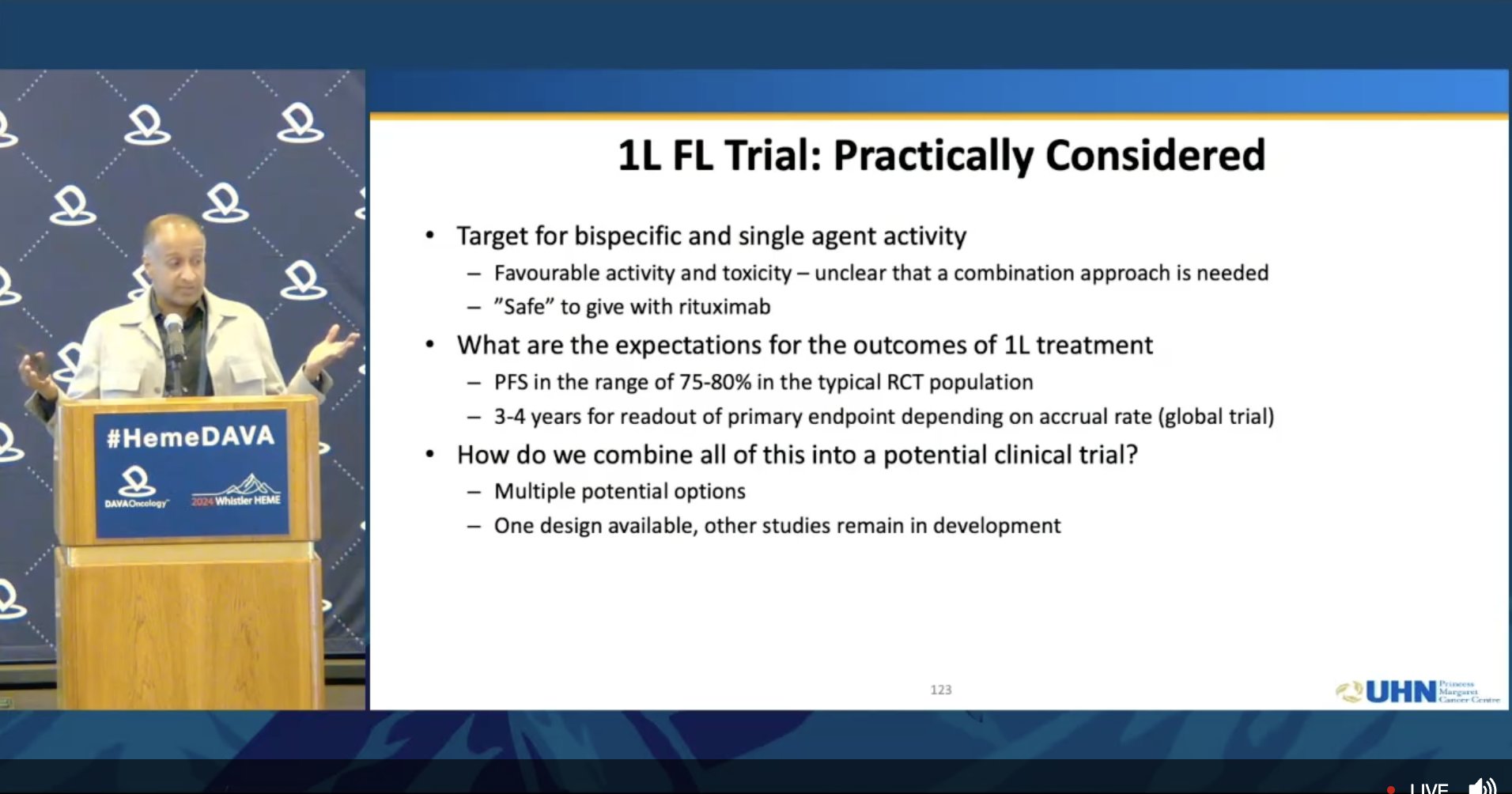
Dr. John Kuruvilla discusses the practical considerations for trial design when moving bispecifics to the frontline for follicular lymphoma Particularly in indolent disease like FL, a 1L trial will be challenging due to improved outcomes requiring a very long study follow up.
Lots of great discussions on the future of FL therapy with this panel! ❓How do we improve outcomes for FL patients without increasing toxicity/decreasing QOL? ❓How do we identify the "bad actors" who could benefit the most from an escalated treatment approach?

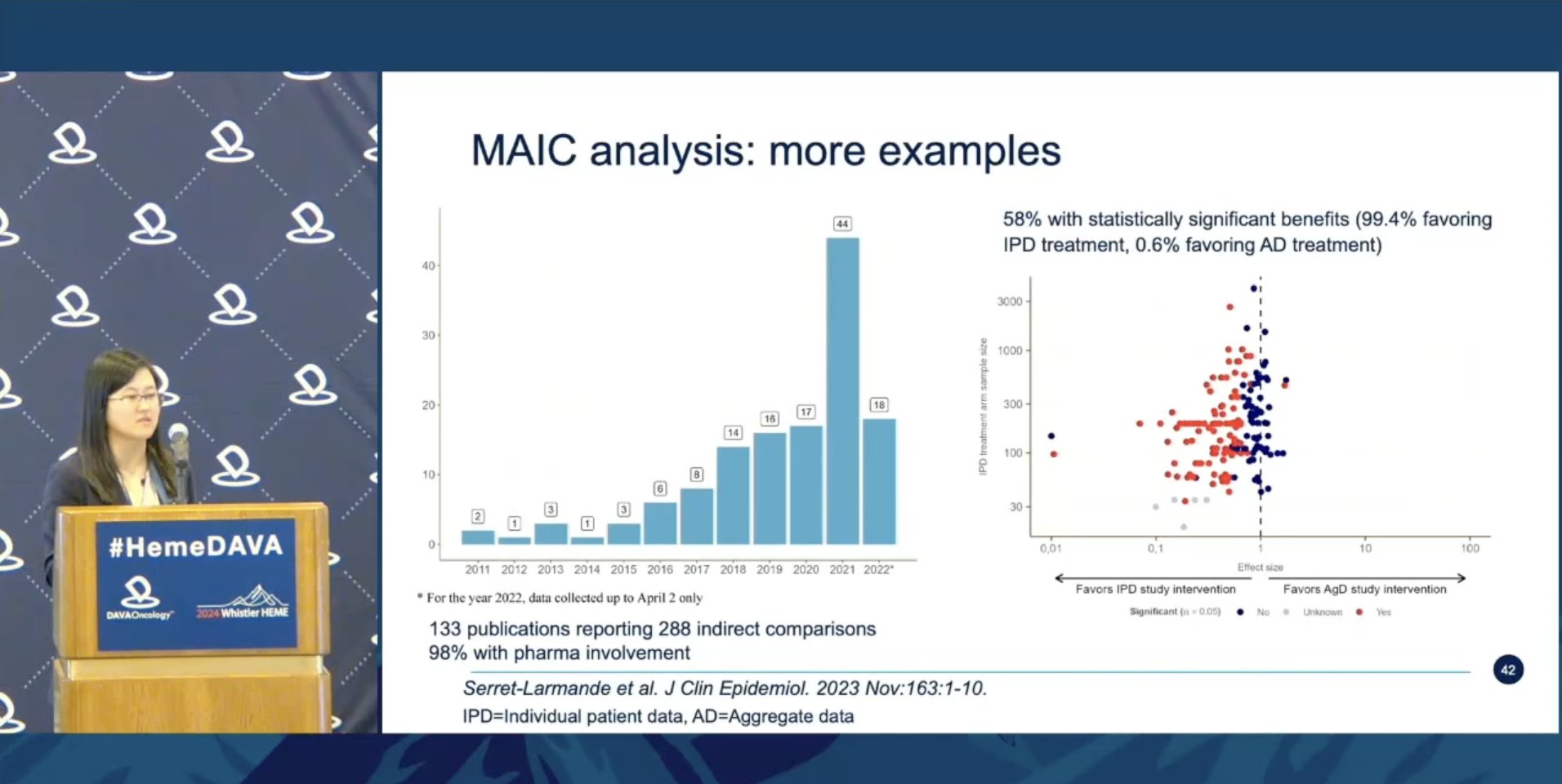
Fascinating! In a review of 288 MAIC analyses, 99.4% of analyses reporting a statistically significant difference favored the individual patient data arm. @DiMengyang concludes that MAIC may be helpful, but fall at Retrospective/Prospective Cohort in the pyramid of evidence.
Thank you to our faculty who made the trip as well as our industry partners who make these meetings possible.


